

Organization: Health Canada Date published: 2018-10-01
Date Adopted: 2017/10/06Guidance documents are meant to provide assistance to industry and health care professionals on how to comply with governing statutes and regulations. Guidance documents also provide assistance to staff on how Health Canada mandates and objectives should be implemented in a manner that is fair, consistent and effective. Guidance documents are administrative instruments not having force of law and, as such, allow for flexibility in approach. Alternate approaches to the principles and practices described in this document may be acceptable provided they are supported by adequate justification. Alternate approaches should be discussed in advance with the relevant program area to avoid the possible finding that applicable statutory or regulatory requirements have not been met. As a corollary to the above, it is equally important to note that Health Canada reserves the right to request information or material, or define conditions not specifically described in this document, in order to allow the Department to adequately assess the safety, effectiveness or quality of a therapeutic product. Health Canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented.
The Food and Drugs Act (FDA) sets out the legislative framework under which medical devices are regulated. The Medical Devices Bureau is responsible for administering the requirements within Part 3 of the Medical Devices Regulations (herein referred to as the Regulations) that govern the sale and importation of a medical device for investigational testing involving human subjects. Manufacturers and importers must meet the regulatory requirements therein including requirements outlined in subsection 83(1) of the Regulations in order to receive authorization from Health Canada to sell a device to a qualified investigator for the purpose of conducting investigational testing.
To provide assistance to manufacturers and importers in preparing the documentation necessary to obtain an authorization for the sale or importation of a medical device under an Investigational Testing Authorization (ITA), while assuring the protection of research subjects, and promoting excellence in research and development in Canada. In doing so, Health Canada endeavors to improve access to new and innovative medical device technologies for Canadians.
Manufacturers and importers are required to submit ITA applications to Health Canada in order to sell or import a medical device for the purpose of conducting investigational testing in human subjects.
Health Canada’s expectation is that manufacturers follow the principles of the Declaration of Helsinki and the Tri-Council Policy Statement (2nd Edition): Ethical Conduct for Research Involving Humans (2014), and conform to Good Clinical Practices (GCP) as set out by ISO 14155 - Clinical investigation of medical devices for human subjects. ISO 14155 is generally consistent with the definitions and requirements of the Regulations. Where inconsistencies exist, the Regulations take precedence.
Research Ethics Boards (REBs) play an important role in the oversight of the conduct of investigational testing, and REB information is required for all clinical trials involving medical devices by Part 3, section 81 of the Regulations. Health Canada will issue a “Letter of Authorization” for investigational testing of Class III and IV medical devices, if the application meets the requirements stated in Part 3 of the Regulations, although REB approval may not be available at the time the ITA application review has been completed. Manufacturers and importers are reminded that this information is required prior to study initiation, and for Class III and IV medical devices, the REB approval letter must be submitted to Health Canada as soon as it becomes available.
Manufacturers and importers are requested to submit ITA applications in the “non-eCTD electronic-only” format and follow the structure presented in Appendix 4.
This guidance document is intended to assist manufacturers and importers with organizing and submitting an ITA application to conduct investigational testing of a Class II, III or IV device, by the manufacturer, an academic institution, a health care facility or a contract research organization. It also provides details on the responsibilities of manufacturers and importers when conducting investigational testing using Class I devices. Further, it will assist investigators and institutions involved in the investigational testing of medical devices in Canada to understand their roles and responsibilities in this process.
This document is not applicable to the investigational testing of in vitro diagnostic devices (IVDDs) in Canada. Manufacturers and importers are referred to the guidance document titled Preparation of an Application for Investigational Testing - in vitro Diagnostics (GD010) available on the Government of Canada website (refer to Appendix 2).
This guidance document supersedes the previous guidance document titled: Preparation of an Application for Investigational Testing - Medical Devices (GD009/Rev00-MDB V3 dated 1999-02-22).
Investigational testing of medical devices in human subjects has become a growing area for research and development in Canada since the establishment of the new Medical Devices Regulations in May, 1998.
This guidance document has been updated from the previous guidance document published in 1999 to add clarity, respond to stakeholder concerns and address topics such as: the use of recognized standards under the Regulations, drug-device combination products, the use of unlicensed devices in drug studies, timing of REB approval for Class III and IV medical devices, investigator-sponsored investigational testing, stages of product development, revisions to an investigational testing protocol, and problem reporting. Moving forward, Health Canada intends to review this guidance document regularly to respond to stakeholder concerns and a rapidly changing medical device landscape within Canada.
CTA Clinical Trial Application (drugs and biologics) CTD Common Technical Document GCP Good Clinical Practices IA Investigator Agreement IB Investigator’s Brochure ICF Informed Consent Form IFU Instructions for Use IT Investigational Testing ITA Investigational Testing Authorization IVDD In-Vitro Diagnostic Devices ISO International Organization for Standardization MDB Medical Devices Bureau MDCU Medical Devices Compliance Unit MDPR Medical Devices Problem Reporting RCT Randomized Controlled Trial REB Research Ethics Board TPD Therapeutic Products Directorate
Most of the definitions listed below were taken from the Regulations and ISO 14155 Clinical investigation of medical devices for human subjects - Good Clinical Practice.
In most cases, general enquiries can be answered by email or by phone. In order to informatively and accurately respond to a sponsor’s questions regarding an ITA application, and provide more in-depth advice, manufacturers and importers are encouraged to request a pre-ITA application meeting, particularly for novel Class III and IV devices and combination products. Such a consultation may be useful and can assist applicants with the completion of applications to facilitate a timely regulatory decision.
The purpose of these pre-ITA application meetings is to provide the manufacturer or importer an opportunity to present relevant data and discuss concerns and issues regarding product development. It also gives Health Canada an opportunity to provide guidance and highlight potential deficiencies or concerns with the proposed investigation. Manufacturers and importers may invite the qualified investigators who will be involved in the proposed investigation in Canada to attend the meeting.
Requests for a consultation meeting or pre-ITA application meeting should be submitted in writing by the manufacturer or importer to the Investigational Testing Division of the Medical Devices Bureau at: it-ee@hc-sc.gc.ca.
Requests should be submitted in the form of a cover letter proposing three (3) dates and times suitable for the meeting. The cover letter should be accompanied by the following information:
Health Canada will acknowledge the request for a meeting in a timely manner. If Health Canada agrees to hold the meeting, the acknowledgement will indicate the date that the pre-ITA application information package is to be provided, the meeting date and location, as well as the list of attendees. Please note that Health Canada reserves the right to propose a teleconference meeting or to address the questions by email correspondence, instead of a face-to-face meeting.
The information package should be submitted in electronic format, and should contain:
Should the pre-ITA application package be found to be deficient, the manufacturer or importer may be requested to reschedule or postpone the meeting to allow the manufacturer or importer to assemble a more complete package. Please note that Health Canada reserves the right to modify or truncate the proposed agenda as it sees fit to better achieve the stated goals of the meeting.
The manufacturer or importer is responsible for sending Health Canada a written record of the discussion and conclusions of the meeting within fourteen (14) days after the meeting. All final records of this consultation will be kept on file.
A copy of the record of discussion and conclusions approved by all parties in attendance at the meeting should be included in the subsequent ITA application.
Under the Regulations, only manufacturers and importers can apply for an authorization to conduct investigational testing on human subjects in Canada. In either case a senior official of the manufacturer must complete and sign the Application Form. However, an investigator/clinician may act as a regulatory correspondent, if authorized by the manufacturer. Based on the requirements of section 82, supporting records as detailed in section 81 paragraphs (a) to (k) applicable to the device classification and section 86 (labelling) of the Regulations must be submitted. Health Canada expects that these records be submitted in an editable electronic copy Footnote 12 .
In general, as a sponsor proceeds through the stages of product development, more evidence in support of the use of the product for more specific clinical indications will be gathered. For first in human and early feasibility studies, there may be an emphasis on pre-clinical/nonclinical testing and a thorough risk assessment compared to traditional feasibility and pivotal studies, where additional clinical data supportive of the proposed indications and patient population may be expected. However, this may also be case dependent and for this reason, it is difficult to outline specific criteria of data required for each of the above stages. Please consult our office for additional information concerning your specific case/circumstances.
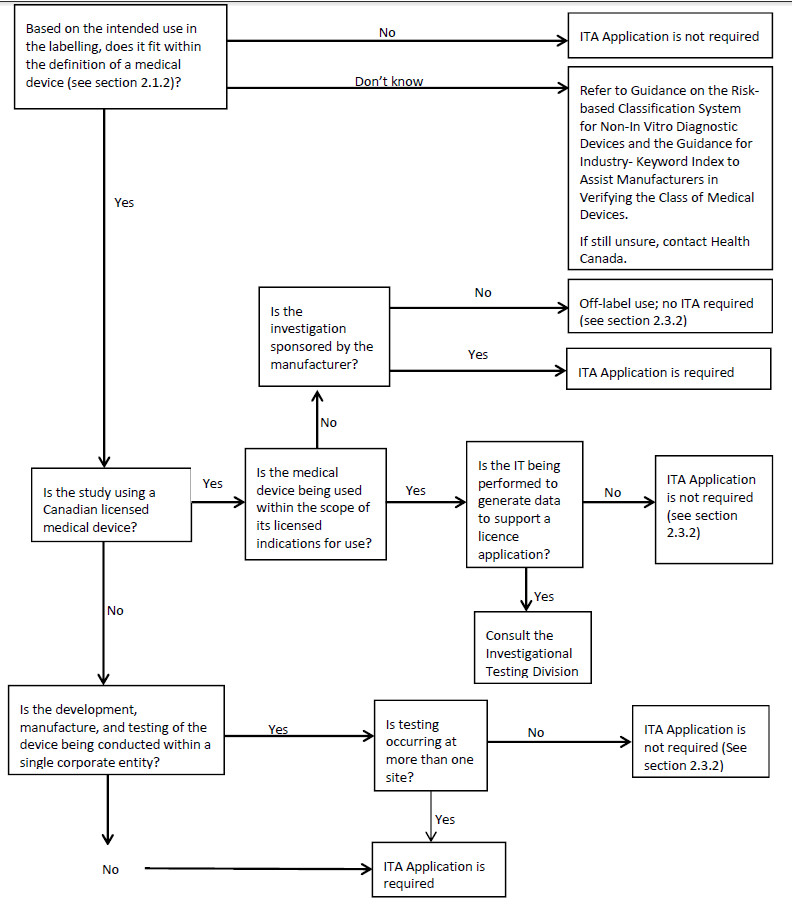
An ITA application is required for all unlicensed class II, III, and IV medical devices (as defined under the Food and Drugs Act) that will be imported and/or sold in Canada for the purpose of investigational testing involving humans (refer to Appendix 3), so that Health Canada can determine whether:
The classification of a medical device product is based on the manufacturer’s representation of the device in the labelling, and how it will be used in the study. Additional information to assist with classification can be found in the Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs) and the Guidance for Industry - Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices (refer to Appendix 2).
Manufacturers may contact the Investigational Testing division of Health Canada for additional guidance, and to determine whether an ITA application is required at: it-ee@hc-sc.gc.ca.
An ITA is not required if there is no sale of the medical device, based on the definition of sale under the Food and Drugs Act. Consequently, if the development, manufacture, and testing of the device are conducted within a single corporate entity, no sale has occurred and the requirements of the Regulations do not apply. The testing must be limited to use on-site (at the corporate entity), and solely by the legal manufacturer.
An ITA is not required for the conduct of a study using a licensed device according to its licensed indications for use (whether it is manufacturer or clinician sponsored).
A licensed device to be used in a manufacturer-sponsored study intended to generate data to support a new indication for use will require an ITA application.
However, licensed medical devices used in clinician-sponsored investigations that are not initiated by the manufacturer, and are not intended to generate data to support a licensing application do not require an ITA. Likewise, licensed medical devices used in clinical investigations outside the scope of the authorized indications for use and not sponsored by the manufacturer do not fall under the Regulations; this is considered off-label use.
If the investigator initiated trial involves an unlicensed device, an ITA would be required. In these cases, the investigator must obtain the cooperation of the manufacturer, who must be the official applicant and signatory for the ITA application. Regulatory correspondence and clinical oversight can be delegated by the manufacturer who is the legal sponsor of the clinical investigation.
Parallel applications for an ITA and a medical device licence should not be submitted, where the ITA application is not intended to gather additional safety and/or effectiveness information to support a device licence, but instead is used to gain expedited market access.
A pulse sequence is considered a component or accessory of a Magnetic Resonance Diagnostic Device (MRDD) system, which meets the definition of a Class II medical device. It is a software-based set of instructions that control how the device operates in order to capture images using variables such as echo time, repetition time, flip angle, field of view, resolution, pulse bandwidth, and post processing. Clinical studies involving work-in-progress (WIP) pulse sequence software protocols for MRDDs are often exchanged between manufacturers and researchers for the purpose of testing the WIP pulse sequence within the licensed MRI device parameters.
In the context of the regulatory review and for international alignment purposes, Health Canada has taken the position that investigators are no longer required to obtain approval from the department prior to starting a trial using a WIP pulse sequence when the conditions below are met. Before initiating a trial, investigators should:
A pulse sequence operating outside the limits governed by the licensed MR scanner or use of a WIP with an unlicensed scanner would still require an ITA.
An investigator that is unsure if their trial meets these conditions is encouraged to contact Health Canada to determine whether an ITA is required.
WIPs which do not require an ITA must not exceed the following listed parameters:
| Population | Main static magnetic field greater than (tesla) |
|---|---|
| adults, children, and infants aged > 1 month | 8 |
| neonates i.e., infants aged 1 month or less | 4 |
| Site | Dose | Time (min) Equal to or greater than: | SAR (w/kg) |
|---|---|---|---|
| whole body | averaged over | 15 | >4 |
| head | averaged over | 10 | >3.2 |
Gradient fields rate of change Any time there is a rate of change of gradient fields (dB/dt) sufficient to produce severe discomfort or painful nerve stimulation. Sound pressure level Peak unweighted sound pressure level greater than 140 dB, and a-weighted root mean square (rms) sound pressure level greater than 99 dBA with hearing protection in place.
All applications must be submitted electronically in accordance with current format for the submission of ITA applications, as specified in the Notice - Applications for Investigational Testing Authorization (ITA), for Medical Devices, in the “Non-eCTD Electronics-Only” Format (refer to Appendix 4). You may also refer to the Guidance Document: Preparation of Regulatory Activities in “Non-eCTD Electronic-Only” Format for detailed guidance on filing medical device regulatory activities and subsequent transactions, in the “non-eCTD electronic-only” format (refer to Appendix 2). It should be noted that Health Canada no longer accepts paper copies of applications. Applications should be submitted by e-mail to devicelicensing-homologationinstruments@hc-sc.gc.ca, or sent by mail to the Health Canada mailing address (refer to Appendix 1) on a CD or DVD if it is too large to be sent by e-mail (20 MB). Applications sent by email should include a subject line that clearly distinguishes it as an ITA application. Requests for revised ITA applications should be clearly marked as such and include the previously assigned application number. ITA applications are not subject to a fee.
Applications sponsored by an investigator or a third party (e.g., a funding agency, a drug manufacturer, another device manufacturer, or a health care institution), must be signed by a senior official of the manufacturer of the unlicensed device. Regulatory correspondence and clinical oversight can be delegated to the clinical investigator, manufacturer, their regulatory agent, or contract research organization. In this case, the official who has delegated authority assumes the responsibilities for the importer to submit the application.
Manufacturers are encouraged to register their clinical investigations on a publicly accessible registry which accepts international clinical trial information and which is recognized by the World Health Organization (WHO). ClinicalTrials.gov and Current Controlled Trials International Standard Randomised Controlled Trials Number Register are acceptable. Information on investigational testing of medical devices in Canada can be obtained directly from the manufacturer, or by consulting one of the registries.
In the case where one study protocol contains multiple unlicensed devices by different manufacturers, each manufacturer must submit a separate ITA application (form and submission). A manufacturer may only list the devices they manufacture on their application form. As an option, one manufacturer may submit a complete ITA application which contains all “Institutional Information” and “Study Documents” contents. These sections may be referenced in the other manufacturer(s) ITA application(s), however each manufacturer must still submit all device specific data and/or testing for the devices that they manufacture. The executive summary or cover letter should indicate the rationale for using the unlicensed device in conjunction with the other devices under the same study protocol as well as reference whether an application is pending or to be submitted for the other devices. To the extent possible the applications should be submitted at the same time. Authorization will not be granted until all applications have been submitted and found to satisfy the regulatory requirements.
International drug clinical trials sometimes use ancillary devices that are not licensed in Canada. For Clinical Trial Applications (CTAs) that involve the use of an unlicensed Class II, III, or IV medical device, a separate ITA application and CTA must be filed and each should be authorized before the trial can commence in Canada. These applications can be filed concurrently.
In this case, the manufacturer of the device must sign the ITA application form, and regulatory correspondence is usually delegated to the drug sponsor. For Class III and IV devices, the pre-clinical information is submitted to Health Canada by the device manufacturer, and the study protocol and Informed Consent Forms (ICFs) are submitted by the sponsor of the drug study. Although the study protocol and the ICF are relevant to the pharmaceutical/biologic study, these documents should be filed for review with the corresponding ITA application. The CTA number should be provided at the time the ITA application is submitted (or as soon as it becomes available) as well as information on when it was submitted, and a No Objection Letter (NOL), if one has been issued.
For the investigational testing of a drug/biologic-device combination product, ITAs or CTAs must be submitted to the lead Directorate within Health Canada, depending on the principal mechanism of action and hence the classification of the product. Refer to the guidance document entitled Drug/Medical Device Combination Products Policy (refer to Appendix 2).
Authorization for the sale and importation of all investigational products to be used within a medical device clinical investigation or a drug/biologic clinical trial must be obtained prior to the initiation of the clinical trial or implementation of the protocol amendment. Therefore, separate ITAs or CTAs (drug or biological) must be submitted for investigational products that are not regulated as combination product.
Health Canada will be responsible for communicating all regulatory decisions to the sponsor or manufacturer.
To facilitate the timely evaluation of ITA applications, it is recommended that the manufacturer or importer provide a detailed cover letter, an executive summary, a table of contents and the required sections based on the class of the medical device to support the intent of the application. The application should be organized as outlined in Appendix 4.
For cases where multiple investigations are being conducted using the same medical device, a separate ITA application is required for each study protocol. When submitting an ITA for a new protocol using a previously authorized device and no changes have been made to the device since its last authorization, the device information in the new ITA application should be cross-referenced to the previous ITA application. In such cases, the cross-references should appear in the new ITA application package under the appropriate folder where the information would be expected to be found, and should include the ITA application number, date of information submitted, and section/folder number of the referenced previous ITA application. In all cases, when the device information from the previous ITA remains unchanged, a cross-reference should be used and the information should not be resubmitted.
When cross-referencing device information to a previous ITA where the investigation has been completed and closed, the results of the previously authorized study should be provided, if available at the time of submitting the ITA application.
Information in the document may be in either French or English. Material in a foreign language must be accompanied by an English or French translation.
The manufacturer or importer is responsible for informing Health Canada of changes in regulatory contact information. Changes to the assigned regulatory contact should be submitted by the current regulatory contact or a senior official in the company and addressed to: devicelicensing-homologationinstruments@hc-sc.gc.ca.
Information that must be submitted for an ITA application depends on the risk class of the device and is outlined in Table 1. The manufacturer or importer must possess records that contain all of the information and documents required under section 81 of the Regulations. For some Class II devices, Health Canada may, under section 84, request additional information that is normally required for a Class III or IV device. It should be noted that the evidence required to demonstrate safety, effectiveness, or performance for devices to be used in investigational testing is different from the evidence required for a licence application.
Applications must meet all regulatory requirements, have a favourable benefit to risk ratio, and address potential risks in a complete patient ICF.
Manufacturers should follow the principles of the Declaration of Helsinki and the Tri-Council Policy Statement (2nd Edition): Ethical Conduct for Research Involving Humans (2014), and conform to Good Clinical Practices (GCP) as set out by ISO 14155 - Clinical investigation of medical devices for human subjects. ISO 14155 is generally consistent with the definitions and requirements of the Regulations. Where inconsistencies exist, the Regulations take precedence.
Conformity with GLP is strongly recommended, and animal studies should be conducted with scientific rigour similar to that in human trials.
Applications for devices that were previously authorized for investigation under a different study protocol can be cross-referenced for device specific information. The results of the previously authorized study should be provided, if available at the time the application is submitted.
Table 1 lists ITA application requirements for different device classes.
There is no requirement to obtain an ITA for a Class I medical device. Subsection 80(3) of the Regulations permits a manufacturer or importer of a Class I medical device to sell the device to a qualified investigator for the purpose of conducting investigational testing. The manufacturer or importer is still required to possess all the records and information detailed in section 81. Institutional requirements and Research Ethics Board (REB) approval apply.
The information required under subsections 81(a), (b), (h), (i), and (j) must be submitted. The manufacturer or importer must possess records that contain all the information required by section 81 of the Regulations. It is not required that evidence of REB approval is provided to Health Canada, but it must be obtained prior to study initiation, in accordance with institutional policies.
Class III and IV
The information required under subsections 81 (a) to (k) must be submitted. However, a “Letter of Authorization” for investigational testing may be issued, prior to the receipt of REB approval and finalization of the qualified investigators and investigational sites, provided that the name and accompanying information (name, address, cv, and signed attestation) of at least one qualified investigator at one investigational site is identified at the time of filing.
Class II, III and IV
Cover Letter & Executive Summary
Include a short Cover Letter to explain the reason for the application and indicating the applicant’s name, any cross-references to previous ITAs and/or device licences, as well as whether the application is a new or revised ITA (with a reference to the ITA number). In addition, a one or two page Executive Summary of the application should be submitted (refer to Appendix 4).
Table of Contents
Include a Table of Contents listing the contents of the application and their location.
Application Form
Include a copy of the completed ITA Application form (Application for Investigational Testing Authorization, refer to Appendix 2).
Pre-submission Correspondence
Include copies of any decisions from pre-submission correspondence that occurred with Health Canada, including minutes from pre-ITA meetings, application enquiries, classification decisions, as well as correspondence with other bureaus in Health Canada.
Class II, III and IV
Manufacturer or Importer Identification
Provide the complete name and address of the device manufacturer and importer, if applicable, including contact names, e-mail addresses, fax and telephone numbers. The name of the legal manufacturer must be consistent with the information found on the device labelling.
Class II, III and IV
Device Identification
Provide the name of the device and the device identifiers, as they appear on the label. This includes any component, part or accessory that is part of the device.
Specify the risk classification of the device based on the Classification Rules set out in Schedule 1 of the Medical Devices Regulations and on the manufacturer’s representation in the labelling. For assistance in determining the risk class please refer to the Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs) and the Guidance for Industry- Keyword Index to Assist Manufacturers in Verifying the Class of Medical Devices. If, after reviewing these documents assistance is still required you may contact, meddevices-instrumentsmed@hc-sc.gc.ca.
To confirm the risk classification of the device, copies of device labelling with clear descriptions of the device and an intended use/indications for use statement can be submitted to the Device Licensing Services Division at, devicelicensing-homologationinstruments@hc-sc.gc.ca.
The risk classification of a device under investigational testing may differ from that of the device in general sale if, for example, a new indication is being investigated. The risk class assigned to an investigational device may also be different from the classification assigned by Health Canada at the time a future license application is filed if at the time of licensing it is deemed to present a higher or lower risk.
This section should also include the number of units of each individually sold device requested for the conduct of this study in Canada (including the total number of devices used and estimates of the number of devices per site).
Class III and IV
Device Description
This section requires a complete description of the device, hardware and software components, and materials used in its construction and packaging (i.e., physical and chemical characterization, key specifications and performance features, component parts, accessories, patient contact materials, and packaging materials). This description should include good quality colour photographs of the device, its components, parts and accessories and engineering diagrams, where appropriate.
Engineering diagrams of long term implantable devices aid in the determination of dimensions and relative proportions. These should be provided with an original application for investigational testing. Engineering diagrams for other device types, such as electro-medical devices may be requested as additional information if necessary to establish the safety and potential effectiveness of the device in question.
If the device has previously been authorized for investigational testing or granted a medical device licence, provide the authorization number and clearly specify if changes have been made to the device. If so, provide a tabular comparison of the similarities and differences with regard to the intended use, design, key specifications and performance features, hardware, software, accessories, patient contact materials, etc. It is recommended that this comparison be provided in tabular form.
Class III and IV
Design Philosophy
Include a description of the features of the device that permit it to be used for the medical conditions and purposes for which it will be sold by the manufacturer. A brief description of the device’s design philosophy and performance specifications should be provided and linked to the objectives of the proposed investigational testing. References and comparisons with appropriate previous versions or generations of the device should be presented. A tabular format is preferred for this comparison.
This section should include an overview of the purposes and principles of operation for the device and should include a summary of the method of use and operation of the device.
Class II, III and IV
Indications for Use, Intended Use, Contraindications
Include the proposed indications for use and/or intended use as well as any contraindications for the device, as proposed by the manufacturer.
Class III and IV
Marketing History
The marketing history should provide details of the regulatory status of the device in other jurisdictions, the volume of sales by country, a summary of reported problems with the device and details of any recalls in other jurisdictions. Include a summary of Special Access requests made to Health Canada and the outcome of these requests.
Class III and IV
Risk Assessment
The risk assessment is an analysis and evaluation of the risks inherent in the use of the device and the measures adopted to reduce these risks to acceptable levels for the purposes of conducting the investigational testing. The use of ISO 14971-1 (Medical Devices - risk management Part 1: Application of risk analysis) is recommended.
Include a list of all possible hazards and evaluate them against the presumed benefits of the device. Additionally, an indication of the way by which the risks have been reduced to acceptable levels must be provided. The risk analysis should be signed, and the identity of the responsible individual provided.
Class II, III and IV
Previous Studies
The results of any previous research, bench testing, pre-clinical and clinical studies conducted with the device must be provided. These results are useful in assessing the safety and potential preliminary effectiveness of the device for investigational testing.
Examples include physical and mechanical bench testing, biological safety, electrical safety, software validation and verification, biocompatibility, shelf life, sterilization validation and residuals testing, packaging validation, animal studies and clinical studies. Supporting literature and a bibliography should be provided, as applicable.
Evidence of conformity to recognized standards should be provided, as described in the Guidance Document: Recognition and Use of Standards under the Medical Devices Regulations (refer to Appendix 2). A Declaration of Conformity (DoC) to a recognized standard can replace detailed pre-market information to satisfy the regulatory requirements. It should be noted that if conformity is declared to a standard that specifies only test methods (such as ISO 10993 for the evaluation of biocompatibility), or procedures (such as ISO 14971 for application of a risk management system for medical devices), quantitative information on test results is also required.
In the case of Class II devices, a DoC is usually adequate to initially support safety of the device. However, Health Canada reserves the right to request additional information on a case by case basis.
Class III and IV
Alternate Treatments
Provide a description of the methods currently used to diagnose or treat the medical conditions that are the subject of the proposed investigational testing.
Class III and IV
Precautions
Provide information respecting any cautions, warnings, contra-indications and possible adverse effects associated with the use of the device.
Class II, III and IV
Subsection 81(g, h)
Institutional Information
For Class II devices, provide the full name of the investigator(s) as well as the name and full mailing address and primary contact information of each institution where the testing is proposed to be conducted. Investigators who are not actively involved in the implantation or use of the device should not be listed. These institutions and names of the investigators actively involved in the implantation or use of the device will be listed on the authorization specified under subsection 83(2). In addition, for class III and IV devices the curriculum vitae should be provided for each investigator to be included on the authorization letter.
A “Letter of Authorization” for investigational testing may be issued, even if the institutions and names of qualified investigators are not all known at the time of submitting the ITA application. At a minimum, information on at least one of the qualified investigators and investigational sites is required at the time of filing. Additional investigators and sites can be added to the authorization by filing the form, titled “Application for Revised Investigational Testing Authorization”. Please submit the form to, devicelicensing-homologationinstruments@hc-sc.gc.ca.
Class III and IV
Research Ethics Board Approval
For Class II medical devices, although REB approval must be obtained before study initiation, applicants are not required to provide evidence of written approval from the REB to Health Canada (refer to Appendix 5).
For Class III and IV medical devices, applicants are required to provide evidence of written approval from the REB along with study documents referenced in the REB approval to Health Canada prior to study initiation. This approval letter must reference the most current study documents (e.g. protocol and informed consent forms). REB approval should ideally be submitted with the ITA application. However, Health Canada may issue a “Letter of Authorization” for investigational testing, if the application meets the requirements stated in Part 3 of the Regulations and REB approval is not available at the time the ITA application review has been completed.
Prior to study initiation, written REB approval which references the most current study documents must be submitted to devicelicensing-homologationinstruments@hc-sc.gc.ca.
If REB approval references updated study documents, you must submit the following prior to study initiation:
A description of the constitution, roles, and responsibilities of an REB can be found under the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2) and ISO 14155.
Please note that previous REB refusals (Canadian and foreign) should be reported and discussed in your initial application.
Class II, III and IV
Protocol
A protocol in line with the GCP recommendations, as per ISO 14155, should be provided. The protocol should include a version number and date of last modification, for document control purposes. The protocol of the proposed investigational testing should contain the following information, in easily identifiable subsections.
Background information describing the disease or condition to be treated, prevalence, diagnostic criteria, and current treatment, study hypothesis, study objectives, study design, inclusion and exclusion criteria, number of devices and study subjects required, as well as detailed information on the duration of the investigation and the follow-up period for patients.
The methods of assessing the investigational device must be fully described, including the criteria for success or failure of the performance of the device. Provide a copy of all case report forms to be used in the investigation. The proposed methods of data analysis should be described, including the identity of the person or group performing the analysis. Methods of data quality control should be specified. The control group must be fully described, and a justification provided for the type of control group chosen.
Provide a full description of the subject selection, including:
Subjects should be selected to be representative of the population intended to be treated with the device, with appropriate inclusion of children, women, and ethnic groups.
Specify the estimated time frame for the conduct of the study, including the duration of the enrolment phase, the duration of the treatment phase, and the duration of the follow-up phase. Also include the approximate duration from the start of enrolment to the end of follow-up period. This can be provided as part of the protocol or provided separately.
Investigator's Brochure (IB)
A copy of the current IB should be submitted for high risk class III and IV devices. This should be supplemented as appropriate with up-to-date safety, non-clinical and clinical data. The IB containing all information regarding the product to date should be prepared in accordance with ISO 14155.
Informed Consent Form (ICF)
Include a copy of the ICFs to be used in conjunction with the study, including information regarding the risks and anticipated benefits to the patients as a result of their participation in the investigational testing. The ICFs to be used in conjunction with the study should be prepared in accordance with applicable laws governing consent. ISO 14155 and the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2) provide requirements for the ICF. The document should include a version number and date of last modification (for document control purposes) which must be referenced by the most current and relevant REB approval.
Class II, III and IV
Device Labelling
The labelling requirements are described in section 86 of the Regulations. In addition to the name of the device and the name of the manufacturer, the label must include the statements “Investigational Device” and “To be Used by Qualified Investigators Only” and “ Instrument de recherche ” and “ Réservé uniquement à l’usage de chercheurs compétents ” in English and French. It is possible to use alternate phrasing, provided the above meanings are conveyed. This should be included in the Operator’s Manual (or Instructions for Use) and on the package label. For reusable devices, such as capital equipment, a label should be put directly on the device and/or the displayed on the start-up screen of the graphical user interface.
The device label must be in either English or French. This includes the device package labels, outer carton labels, Instructions for Use (IFU), Operator’s Manual, Training Manual, and all advertising brochures intended to be used with the device. Where the directions for use are supplied in only one official language at the time of sale, directions for use in the other official language must be made available by the manufacturer as soon as possible at the request of the purchaser.
For RCTs, the required labelling (device name, manufacturer name, investigational statement) must be affixed to the outer container, and each individual device (study and control) must bear an assigned code, the manufacturer's name, and the investigational use statement in French and English.
The intent of this label is to ensure that the device is not used other than under the study protocol.
Class III and IV
Investigator Agreement(s)
A signed investigator agreement is required for each investigator to be listed on the authorization.
This agreement outlines the responsibilities of the investigator to:
A template of an investigator agreement form is available on the Government of Canada website (refer to Appendix 2). An alternate format is acceptable provided the five (5) conditions described in subsection 81(k) are adequately addressed.
Manufacturers and importers are reminded that at least one qualified investigator along with their investigator agreement is required at the time of filing the ITA. The new form, titled “Application for Revised Investigational Testing Authorization” should be provided to Health Canada for revisions made to study investigators, sites or their agreements, soon as the information becomes available post-initial authorization. Such revisions can be submitted via this form to, devicelicensing-homologationinstruments@hc-sc.gc.ca.
Class II, III and IV
Minimizing Bias
Bias should be minimized by adherence to currently accepted standards of protocol design as described in the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2) and ISO 14155.
Inclusion of women, children, and vulnerable populations
Efforts should be made to maximize compliance with the International Council for Harmonisation (ICH) Document E11 entitled Clinical Investigation of Medicinal Products in the Paediatric Population and the Health Canada Guidance Document: Considerations for Inclusion of Women in Clinical Trials and Analysis of Sex Differences (refer to Appendix 2).
Clinical Trial Design and Statistical Considerations
The clinical trial design will depend on the stage of development of the device and the clinical investigation.
Blinding (Masking) and Randomization are particularly important for reducing bias in pivotal studies.
A comparator arm is recommended whenever feasible. This includes trial designs in which subjects serve as their own control, either concurrently (e.g., split face therapy) or sequentially.
The statistical basis of the study will be considered to ensure that “the objectives of the study will be achieved” under subsection (83(1)(c)).
General guidance on this topic can be found in the ICH documents E8: General Considerations for Clinical Trials, E9: Statistical Principles of Clinical Trials, and E10 Choice of Control Group and Related Issues in Clinical Trials and Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2) (refer to Appendix 2).
Health Canada will issue an authorization under section 83 of the Regulations after a review of the submitted information is deemed to satisfy the requirements of the Regulations.
This letter will be the manufacturer’s legal authorization to import and sell the requisite number of devices to the investigators or institutions listed under the Authorization for use in the referenced study protocol.
The Letter of Authorization will specify the name of the device, the study protocol title, date and version number, the date of the ICF, the objectives of the study, the authorized number of devices to be imported and sold and number of study subjects to be recruited in Canada, as well as the names of the investigators and institutions where testing may be conducted. This information, along with REB approval(s), is required prior to study initiation. Refer to section 2.3.5 for further details.
Section 87 of the Regulations prohibits advertising a medical device that is the subject of investigational testing unless an authorization has been issued, and the advertisement clearly indicates that the device is the subject of investigational testing, and the purpose of the clinical investigation.
It is expected that manufacturers will have implemented a proper quality management system. Evidence of certification to ISO 13485 is not a requirement for an ITA, however it may be used as guidance.
Under section 80 of the Regulations, the manufacturer or importer of a medical device undergoing investigational testing in Canada must maintain records as detailed under section 81 of the Regulations. The Regulations do not cover the document retention period for clinical institutions. This period should be in line with institutional policies and provincial regulating bodies for the practice of medicine Footnote 13 .
Under section 88 of the Regulations the manufacturer, importer and distributor of a medical device undergoing investigational testing in Canada must maintain distribution records as detailed under sections 52 to 56.
The qualified investigator is required to report serious adverse events to Health Canada and to the manufacturer and importer within 72 hours of discovery. This includes cases in which the incident:
For an incident that occurs in Canada, the manufacturers and importers are required to provide a preliminary and a final report in respect of the incident. The preliminary report shall be submitted:
Further details for the information required in the reports, as well as reporting for incidents outside of Canada can be found in sections 59 to 62 of the Regulations. Manufacturers may reference the Guidance Document for Mandatory Problem Reporting for Medical Devices and relevant forms (refer to Appendix 2) for the process of submitting these reports. Health Canada does not currently have a mandatory form specifically for healthcare professionals. In the interim, healthcare professionals can submit their ITA incidents through Health Canada’s website. A copy of the Medical Devices Problem Report Form can be obtained on the Health Canada website.
Manufacturers and importers must have documented procedures in place to handle product complaints and recalls as required by sections 57 and 58, and 63 to 65 of the Regulations. In addition, the appropriate records of these activities must be maintained.
For additional information, consult the guidance document on complaint handling and recalls (Recall Policy, refer to Appendix 2).
The manufacturer’s responsibilities for implant registration, as described in sections 66 to 68, are also applicable (as appropriate) to devices authorized for investigational testing. Implant Registration cards are required for the devices listed under Schedule 2 of the Regulations.
Manufacturers or importers may submit a request for a revised authorization to address changes made to the device, study investigation plan, or institutional information, such as those listed below:
Examples of protocol changes that would require a revised authorization are listed below. When in doubt whether an application is required, sponsors should contact the Investigational Testing Division at: it-ee@hc-sc.gc.ca.
The criteria for issuing a revised authorization are:
A new application is required for substantial study and/or device changes (e.g., new protocol that may bias the data, device design change that alters its functionality, new or changed indications for use) that introduce new risks. The submission of a new ITA application for this purpose requires the cancellation of the current authorization.
All requirements for filing an ITA, as discussed in section 2.3.5 of this guidance document, are applicable to ITA revision requests, with the exception of the REB approval letter (see page 26). All applicable review sections that have changed as a result of the modifications or are required to validate the modified device and/or support its continued safety in the investigation should be re-submitted. Include a cover letter clearly distinguishing what aspects are modified and which remain unchanged, as well as what supporting information has been provided.
If the protocol, ICF, or labelling have been revised, both clean and redlined copies of the modified documents should be provided along with a tabular list of changes, and a rationale that supports each change.
If device changes are being made or new devices are being added, applicable verification and validation studies should be provided (e.g., design, electrical, performance, sterilization, biocompatibility, etc.).
When filing an ITA revision request, an ITA application form is only required if changes have been made to the device details (i.e., device names, catalogue number, addition/deletion of a device) on the letter of authorization. A new copy of the application form is required with a revised section 11 to reflect the changes, and should be signed by the manufacturer.
Applications for revisions to previously authorized ITAs should be submitted in electronic format following the structure under Appendix 4. All correspondence should reference the previously issued ITA number, including the email subject line and Cover Letter. Completed applications are to be submitted electronically in accordance with the current policy for the submission of ITA applications to: devicelicensing-homologationinstruments@hc-sc.gc.ca or by mail to Health Canada’s postal address (refer to Appendix 1).
Modifications made to the device or the investigation that are not deemed to be changes outlined in section 2.5, which are deemed significant for a revision, should also be reported to Health Canada. These changes will be acknowledged in writing, and a revised authorization may or may not be required depending on the nature of the proposed changes.
These changes may be submitted to Health Canada in the form of a Notification, following the “non-eCTD electronic only” format discussed in section 2.3.3. A detailed cover letter describing the change must be included. All applicable folders with modified information must also be included.
Section 85 of the Regulations allows the Minister to cancel an ITA for Class II, III or IV devices, and to stop the sale of a Class I device that has been sold for investigational purposes, as outlined in subsection 85(1) paragraphs (a) to (e) when:
Prior to cancelling an ITA, Health Canada will request information from the manufacturer or importer to substantiate that the conditions set out in subsection 83(1) are still being met. If this information is not submitted, or if it is submitted and a review of the information determines that any of the conditions set out under section 83 are not met and/or any of the conditions set out under subsection 85(1) exist, the authorization will be cancelled by written notice outlining the reasons for the cancellation.
New ITA applications are screened for administrative and scientific content to ensure that the applicable regulatory requirements of sections 81 and 86 of the Regulations have been addressed. A Screening Acceptance Letter is issued if the information is complete. If regulatory deficiencies are identified at screening, a Screening Deficiency Letter is issued which details the deficiencies. The manufacturer or importer is given fifteen (15) calendar days to provide the missing information, after which time the application is rejected (refer to the Policy on Management of Applications for Medical Device Licences and Investigational Testing Authorizations, Appendix 2). The response should include an Executive Summary listing the responses in a question and answer format, as well as all other documents in the appropriate section of the electronic folder structure for ITA applications (refer to Appendix 4).
A Screening Rejection Letter is issued for grossly deficient applications, such as when substantial information which is required under section 81 of the Regulations is missing from the application. This may result from the submission of a Class II application for a medical device determined by Health Canada to be Class III or IV.
A Screening Rejection Letter will also be issued if the manufacturer or importer fails to provide the records required under section 81 within fifteen (15) days of receipt of a Screening Deficiency Letter that will specify the list of deficiencies. If an application is rejected, the applicant will be required to submit a new application with all relevant supporting data; cross-referencing a previously rejected application in a new application is not permitted.
Once the review has been initiated un-solicited information should not be provided without consent from the Investigational Testing Division Manager. The review period for new ITA applications or requests for revised ITAs, other than for combination products, is thirty (30) calendar days from when screening is initiated. If a Screening Deficiency Letter was issued, the thirty (30) day review period begins on the day that Health Canada was in receipt of a complete and reviewable application. For minor administrative revisions (e.g., as outlined in section 2.5 of this guidance) to ITAs, the review period is fifteen (15) calendar days. It should be noted that these are estimated review targets and not default deadlines that result in automatic authorization.
After the review has been completed Health Canada may send a request for additional information as described in section 84 of the Regulations for any missing information required under section 81. The applicant must submit a complete response within sixty (60) calendar days. Upon receipt of the response to the additional information request from the manufacturer or importer, a new thirty (30) calendar day review period will begin.
Should the applicant be unable to provide the requested information within this timeframe, the application may be withdrawn and resubmitted without prejudice within six (6) months. After this time period a new application must be filed. Requests for additional information that have not been responded to within sixty (60) days will be subject to a refusal. Extensions for additional information may be granted on a case-by-case basis.
If the information submitted in support of the ITA or ITA revision is deemed to be satisfactory, Health Canada will issue an authorization or revised authorization.
The ITA application or revised application may be refused if it is determined that:
A Refusal letter will be issued itemizing any and/or all deficiencies.
The manufacturer or importer may appeal a refusal to issue an ITA. Information on the appeal process for decisions made regarding investigational testing applications can be found in the document Management of Applications for Medical Device Licences and Investigational Testing Authorizations (refer to Appendix 2).
In the event of the premature discontinuation of a study in its entirety or at a study site for which an ITA or ITA revision has been issued in Canada, the manufacturer or importer should notify Health Canada as soon as possible, but no later than fifteen (15) calendar days after the date of discontinuation.
This notification should include:
Note: Notification of a premature discontinuation of international sites (due to safety reasons) for which there are ongoing studies with the medical device in Canada, should also be submitted to Health Canada.
The manufacturer may resume the investigational testing in its entirety or at a site that was previously discontinued if the manufacturer submits the following information:
Note: The above information may be submitted as Additional Information and Health Canada will provide acknowledgement of the resumption. When there has been a change to the device, the study protocol or the manufacturing process, the information should be submitted as a request for a revised ITA (refer to section 2.5 of this guidance). The study may resume only when a revised Letter of Authorization has been issued.
Manufacturers and importers are urged to report the completion of the study to Health Canada. Inclusion of a final study report is encouraged (see section 2.8.4 of this guidance). Subsequent to this notification, Health Canada will issue a letter to confirm closure of the study. ISO 14155 provides additional guidance.
After completion of the investigational testing, re-usable devices, such as capital equipment, which are not yet licensed, should be returned to the manufacturer or importer. If the qualified investigator intends to use these devices under a different study protocol, a new application for investigational testing signed by a senior official of the manufacturer must be submitted. Clinical use of unlicensed medical devices other than under an authorised clinical protocol is prohibited.
After closure of the investigational testing, the manufacturer or importer should submit a report of the study to Health Canada even if the investigation was terminated prematurely.
Upon request, or when determined appropriate, Health Canada will publically release certain information about issued ITAs. The primary goal is to provide transparency and improve public access to information on Canadian studies involving medical devices. For ITAs authorized since November 14, 2013 the following information will be released:
For all other information enquirers will be redirected to the manufacturer or importer, or referred to their health care provider.
Medical Devices
Medical Devices Bureau
Therapeutic Products Directorate
Health Products and Food Branch
Health Canada
2nd Floor, 11 Holland Avenue, Tower A
Address Locator: 3002A
Ottawa, Ontario
Canada
K1A 0K9
General Enquiries concerning Investigational Testing:
E-mail: it-ee@hc-sc.gc.ca
Telephone: 613-941-4308
Pharmaceutical Drugs
Office of Clinical Trials
Therapeutic Products Directorate
Health Products and Food Branch
Health Canada
5th Floor, Holland Cross, Tower B
Address Locator: 3105A
1600 Scott Street
Ottawa, Ontario
Canada
K1A 0K9
General Enquiries:
E-mail: hc.oct_bec_enquiries.sc@canada.ca
Telephone: 613-941-2132
The following documents may be useful in the preparation of the application:
The following is the “non-eCTD electronic-only” format for ITA applications referenced in the Notice - Applications for Investigational Testing Authorization (ITA), for Medical Devices, in the "Non-eCTD Electronics-Only" Format. Some of the sections are not applicable to Class II applications. Empty folders must be deleted before filing to Health Canada. Any other supporting documentation that does not fall into one of the pre-defined folders can be provided in an appendix, but should be clearly referenced in the Executive Summary.
Applications will only be accepted in “Non-eCTD Electronic-Only” electronic format, and may be sent by e-mail to: devicelicensing-homologationinstruments@hc-sc.gc.ca. Please be aware that the maximum email size accepted by the corporate email server is twenty (20) MB, and anything larger should be sent by mail (refer to Appendix 1) on a CD or DVD. If the application package is sent on a CD/DVD, ensure that PDF files are no larger than one hundred and fifty (150) MB to ensure they can be accessed efficiently. For more details, including the required organization of information and file details, refer to the Guidance Document: Preparation of Regulatory Activities in the “Non-eCTD Electronic-Only” Format.
Applications must be submitted by the device manufacturer or the importer. However, they may delegate an investigator to serve as the regulatory contact. In the case where the importer or investigator is submitting the application, the manufacturer must sign off the application form (see section 2.3.3 of the ITA guidance document for more details).
Medical devices manufactured and used in a clinical investigation within the same corporate entity are exempt from the requirements of the Medical Devices Regulations. This applies only to cases when the investigation is occurring at a single institution. In the case where the physician is the legal manufacturer of the device, as defined under the Regulations, and the device is being used on-site where the physician practices, and s/he is the only person responsible for conducting the investigational testing, there is no sale occurring as defined under the Food and Drugs Act. The requirements of the Regulations do not apply for these situations and an ITA is not required (see section 2.3.2 of the ITA guidance document for more details).
Changes to the device and/or protocol that maintain the original study objectives and would not be expected to alter the risk-profile or negatively impact the integrity of the data collected can be submitted as a request for a revised ITA. Evidence to support the requirements under section 81 and 86 of the Regulations should be provided for all requirements that are impacted by the proposed changes. Requests should be submitted electronically in accordance with the current policy for the submission of ITA applications to: devicelicensing-homologationinstruments@hc-sc.gc.ca, or by mail to Health Canada’s postal address (see Appendix 1 and section 2.5 of the ITA guidance document for more details).
For a class I device, there is no requirement to apply for an authorization to conduct investigational testing, but the manufacturer must keep a record of the information required by section 81 of the Medical Devices Regulations on file and the study should be conducted in accordance with good clinical guidance (e.g., ISO 14155, GCPs, etc.).
For Class II medical devices, although REB approval must be obtained before study initiation, applicants are not required to provide evidence of written approval from the REB to Health Canada.
For Class III and IV medical devices, applicants are required to provide evidence of written approval from the REB along with study documents referenced in the REB approval to Health Canada prior to study initiation. This approval letter must reference the most current study documents (e.g. protocol and informed consent forms). REB approval should ideally be submitted with the ITA application. However, Health Canada will issue a “Letter of Authorization” for investigational testing, if the application meets the requirements stated in Part 3 of the Regulations and REB approval is not available at the time the ITA application review has been completed.
Prior to study initiation, written REB approval which references the most current study documents must be submitted along with a completed “Application for Revised Investigational Testing Authorization” to devicelicensing-homologationinstruments@hc-sc.gc.ca.
If REB approval references updated study documents, you must submit the following prior to study initiation:
Section 55 of the Regulations requires the manufacturer, importer and distributor to retain the distribution records maintained in respect of a medical device for the longer of:
The Regulations do not cover the document retention period for clinical institutions. This period should be in line with institutional policies and provincial regulating bodies for the practice of medicine.
The qualified investigator is required to report serious adverse events that fall within the scope of Section 59 of the Regulations to Health Canada and to the manufacturer or importer within seventy-two (72) hours of discovery. The manufacturer and the importer of a medical device are required to report to Health Canada incidents that fall under the scope of section 59 of the Regulations within ten (10) days, if the incident has led to the death or a serious deterioration in the state of health of a patient, user or other person, or within thirty (30) days the incident has not led to the death or a serious deterioration in the state of health of a patient, user or other person, but could do so were it to recur. Further details of the reporting requirements are set out under sections 59 to 62 of the Regulations and found in section 2.4.5 of the ITA guidance document.
The ITA issued pertains specifically to the device, protocol and number of patients referenced on the authorization. After the investigation has been completed the device should be returned to the manufacturer or importer, decommissioned or disposed of appropriately. If the qualified investigator intends to use the devices under a different study protocol, a new ITA application must be submitted by the manufacturer or importer.
The most current versions of ISO standards take precedence over this guidance document, unless the terms are defined in the Food and Drugs Act and Medical Devices Regulations.
Clinical Investigation, Investigational Testing, Clinical Trial and Clinical Study are synonyms.
For medical devices, applications must be submitted by the medical device manufacturer.
Dorland’s Medical Dictionary.
Ethics Committee and Institutional Review Board are synonyms.
Investigation site and investigation centre are synonyms.
The terms “investigational medical device” and “investigational device” are used interchangeably.
Although not defined in the Medical Devices Regulations, an individual member of the investigation site team is commonly called “sub-investigator” or “co-investigator”.
The term "sell," as defined in section 2 of the Food and Drugs Act, is not restricted to commercial or monetary sale and includes transactions without consideration, such as devices provided by the manufacturer to the investigator free of charge.
Planned hospitalization for a pre-existing condition, or a procedure required by the Clinical Investigation Plan, without serious deterioration in health, is not considered a serious adverse event.
Reference can be made to Guidance for the Interpretation of Significant Change of a Medical Device for general principles in determining significant changes.
Health Canada will not accept a scanned copy of the records.
While this is not defined in the Medical Devices Regulations for device, the Food and Drug Regulations define a period of 25 years for the retention of records related to drug clinical studies.